Introduction
During the last few decades, research work has been done to investigate the different drugs of abuse in different types of biological samples (urine, saliva, hair, etc). One of the advantages of hair sample is the noninvasive collection.
Our goal for this application note is to develop a sample preparation method for a drug panel in hair using a single operation in LDTD-MS/MS and the Precellys 24 Touch (Bertin Technologies, Montigny-les-Bretonneux, France).
A proper sample preparation protocol is critical for MS-based analysis workflows. Indeed, the quality and reproducibility of the drug extraction can strongly influence MS results. This requires choosing an optimal protocol for the sample preparation step. The 3D-beating technology is considered the gold standard for sample pulverization. For this reason, Bertin Technologies has chosen 3-dimensional bead-beating technology to power the Precellys 24 Touch homogenizer.
LDTD-MS/MS offers specificity combined with an ultra-fast analysis for an unrivaled screening method. To develop this application, we focused on performing a quick and simple sample preparation. Twenty drugs are analyzed simultaneously with quantitative screening results obtained in less than 8 seconds per sample. Each drug has been screened based on the industry cut-offs required.
Sample Preparation Method
Sample Collection
Ten milligram hair samples (approximately 1 cm length) were transferred into the Precellys MK28R 2mL lysing kit (2 mL reinforced empty vials with 2.8 mm stainless steel beads from Bertin Technologies, Montigny-le-Bretonneux, France, ref : P000917-LYSK0-A.0)
Decontamination and Pulverization
To remove undesired contaminants from the hair surface, 1 mL of methanol was added to the samples, and it was soaked for 5 minutes at room temperature. The washing solution was discarded then samples were dried at 60°C / convection for 30 minutes. Samples were pulverized into a fine powder (3X 60sec at 6500 rpm, 15 seconds pause time) using Precellys 24 Touch system (Figure 3).
Sample Extraction
Samples were extracted as follows:
- Add 1 mL of the internal standard solution in a 2 mL vial.
- For the screening curve: 10 µL of working solution of the standard were added.
- Vials were capped, mixed and incubated at 60°C for 1h45 followed by 15 minutes in the sonication bath.
- Centrifuge for 5 minutes.
- Mix 6.5 µL of desorption buffer with 20 µL of upper layer phase
- Spot 6 µL of the mixture onto a LazWell™96 plate
- Dry 6 minutes at 40°C

LDTD®-MS/MS Parameters
LDTD
Model: Luxon S-960, Phytronix
Carrier gas: 6.0 L/min (air)
Laser pattern:
- 6-second ramp to 55% power
- Hold 2 seconds at 55% power
MS/MS
MS model: Q-Trap System 5500, Sciex
Scan Time: 5 msec
Ionization: APCI
Analysis Method: Positive MRM mode
| Drugs | Transition | CE |
|---|---|---|
| Meprobamate | 219.1 → 158.1 | 10 |
| Carisoprodol-d7 | 268.2 → 183.2 | 10 |
| Nordiazepam | 271.1 → 140.1 | 27 |
| 7-Aminoflunitrazepam | 284.0 → 226.0 | 50 |
| Diazepam | 285.1 → 154.1 | 32 |
| 7-Amino Clonazepam | 286.1 → 222.2 | 30 |
| Oxazepam | 287.1 → 241.1 | 30 |
| 7-Amino Clonazepam-d4 | 290.1 → 226.0 | 30 |
| 7-Amino Flunitrazepam-d7 | 291.0 → 230.0 | 50 |
| Oxazepam-d5 | 292.1 → 246.1 | 30 |
| Temazepam | 301.1 → 255.1 | 25 |
| Temazepam-d5 | 306.1 → 260.1 | 25 |
| Zaleplon | 306.1 → 264.1 | 30 |
| Zolpidem | 308.1 → 236.1 | 35 |
| Alprazolam | 309.1 → 274.1 | 35 |
| Alprazolam-d5 | 314.1 → 286.1 | 35 |
| Clonazepam | 316.0 → 214.0 | 50 |
| Lorazepam | 321.0 → 275.0 | 30 |
| (Alpha) Hydroxyalprazolam | 325.1 → 205.1 | 54 |
| Midazolam | 326.1 → 291.1 | 35 |
| Clozapine | 327.1 → 270.1 | 30 |
| 2-Hydroxyethylflurazepam | 333.1 → 194.1 | 30 |
| Alpha-OH-Midazolam | 342.2 → 203.1 | 35 |
| Etizolam | 343.1 → 314.0 | 25 |
| (Alpha) Hydroxytriazolam | 359.0 → 331.0 | 36 |
| Flurazepam | 388.1 → 315.0 | 27 |
Results and Discussion
Initial Cut-Off Test (pg/mg hair)
A screening cut-off of 500 pg/mg hair is reached for 7-aminoflunitrazepam, 7-aminoclonazepam and nordiazepam. For all other drugs, a screening cut-off of 250 pg/mg hair is obtained.
Precision
Spiked samples around the decision point (50% cut-off: QC-L, cut-off: CO and 200% cut-off: QC-H) and blank solutions are used to validate the precision of the method. The peak area against the internal standard (IS) ratio was used to normalize the signal. Replicate extractions are deposited onto a LazWell™ plate and dried before analysis.
The following acceptance criteria were used:
- Each concentration must not exceed 20% CV
- The mean concentration ± 2 times the standard deviation must not overlap with other concentrations at the cut-off.
For the inter-run precision experiment, each fortified sample set is analyzed in triplicate on five different days. Table 2 shows the inter-run precision results. No overlapping at the cut-off is observed for 2-Hydroxyethylflurazepam and the %CV was below 20%. Similar results are obtained for the other drugs in the panel.
| QC-L | CO | QC-H | |
|---|---|---|---|
| Conc (pg/mg hair) | 125 | 250 | 500 |
| N | 15 | 15 | 15 |
| Mean (pg/mg hair) | 123.7 | 246.2 | 505.6 |
| SD | 17.5 | 15.2 | 21.3 |
| %CV | 14.1 | 6.2 | 4.2 |
| Mean – 2SD (pg/mg hair) | 88.8 | 215.7 | 463.1 |
| Mean + 2SD (pg/mg hair) | 158.7 | 276.7 | 548.2 |
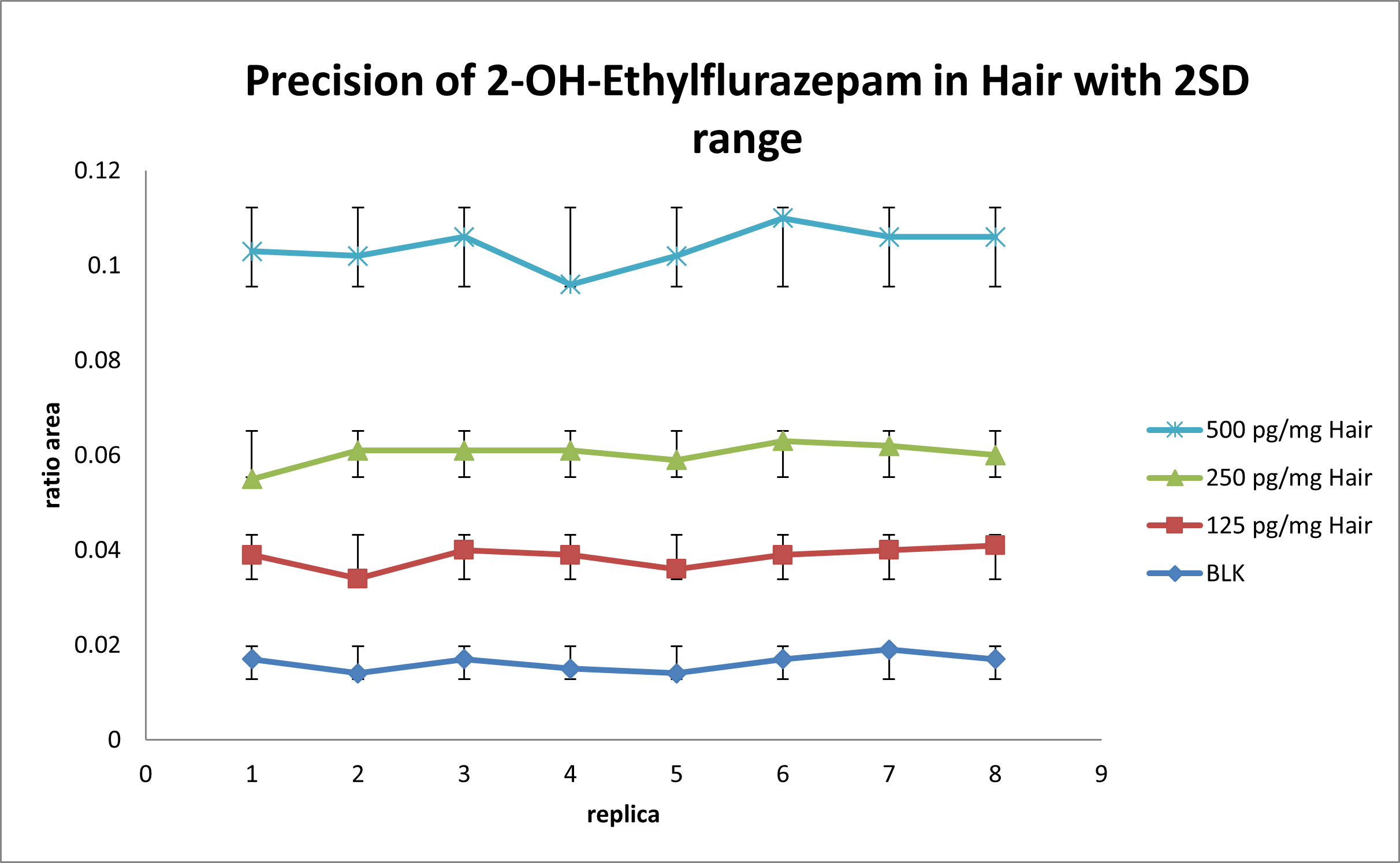
For the intra-run precision experiment, each fortified sample is extracted and analyzed in 8 replicates. Area ratio results are plotted using the ± 2 STD error bars. Figure 4 shows the intra-run results for 2-Hydroxyethylflurazepam. No overlapping is observed for each concentration and the %CV was below 20%. Similar results are obtained for the other drugs in the panel.
Multi-Matrix Validation
Hair samples are collected from ten different volunteers. Samples are screened to verify the presence of each analyte (all samples were negative). Drugs are spiked 200% cut-off (QC-H) and screened as unknown to verify the method sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy.
|
Spiked Sample |
|||
|---|---|---|---|
| Yes | No | ||
| LDTD-MS/MS | Yes | TP (True positive) | FP (False positive) |
| No | FN (False negative) | TN (True negative) | |
Where:
Sensitivity: (TP / (TP + FN))
Specificity: (TN / (TN + FP))
PPV: (TP / (TP + FP))
NPV: (TN / (TN + FN))
Accuracy: ((TP+TN) / (TP + FN+TN+FP))
Table 3 shows the analysis result of non-spiked/spiked samples for 2-Hydroxyethylflurazepam.
| |
LC-MS/MS | ||
|---|---|---|---|
| Yes | No | ||
| LDTD-MS/MS | Yes | TP=10 | FP=0 |
| No | FN=0 | TN=10 | |
Validation results are reported in Table 4 for 2-Hydroxyethylflurazepam. Similar results are obtained for the other drugs.
| Parameters | 2-Hydroxyethylflurazepam |
|---|---|
| Sensitivity (%) | 100 |
| Specificity (%) | 100 |
| PPV (%) | 100 |
| NPV (%) | 100 |
| Accuracy (%) | 100 |
Conclusion
The Precellys®24 Touch can be successfully used to pulverize hair samples for mass spectrometry drug analysis. The Precellys 24 Touch combined with the Luxon Ion Source® and Sciex Q-Trap 5500 mass spectrometer system allows ultra-fast (8 seconds per sample) benzodiazepine panel screening in hair using a simple sample preparation method.