Introduction
The rapid quantification of free estrone in serum can be useful to monitor estrogen levels and help explain abnormal menstrual cycles, symptoms of menopause, abnormal or heavy bleeding, infertility or any other hormonal alterations.
Our goal for this application note is to develop a diagnostic tool to rapidly do the quantification of free estrone in serum, which will allow the rapid diagnosis of abnormal disorders.
LDTD®-MS/MS offers specificity combined with an ultra-fast analysis for an unrivaled quantification method. To develop this application, we focused on performing an efficient sample preparation, which will increase the speed of the analysis process and confirm diagnoses more quickly.
Sample Preparation Method
A stock solution of Estrone was prepared in methanol at 1 mg/mL. Then, a bovine serum albumin solution (20 mg/mL in PBS) was spiked to generate a calibration curve and QCs.
Automated Sample Extraction
An automated system (Figure 3) is used to extract the samples using the following conditions:
- 10 µL of Internal standard in methanol
- 400 µL of Sample
- Vortex
- 800 µL of Hexane: Methyl-tert-butyl ether (90:10)
- Vortex and centrifuge (3000 rpm/4 min.)
- Transfer 500 µL upper layer and evaporate to dryness
- Reconstitute extract with 125 µL Methanol:Water (1:1)
- Spot 6 µL of mixture on a LazWell™96 plate
- Dry 8 minutes at 40°C

LDTD®-MS/MS Parameters
LDTD
Model: Luxon S-960, Phytronix
Carrier gas: 5.0 L/min (air)
Laser pattern:
- 3-second ramp to 45% power
- 2-second hold
MS/MS
MS model: QTrap® System 5500, Sciex
IonSpray Voltage: -5500
Temperature: GS1 and GS2 set to zero.
CUR: 20
Scan Time: 50 msec
Analysis Method: Negative MRM mode
| Transition | CE (V) | |
|---|---|---|
| Estrone | 269.0 → 145.0 | -50 |
| Estrone-d4 | 273.0 → 147.0 | -50 |
Results and Discussion
Validation Test
Calibration curves ranging from 10 to 250 pg/mL were prepared in Bovine serum albumin (20 mg/mL dilute in PBS buffer). A set of QCs were prepared in same matrix. Replicate extractions were deposited onto a LazWell™ plate and dried before analysis. The peak area against the internal standard (IS) ratio was used to normalize the signal.
Linearity
The calibration curves were plotted using the peak area ratio and the nominal concentration of standards. For the linearity test, the following acceptance criteria was used:
- Linear regression (r) must be ≥ 0.995
Table 2 shows the inter-day correlation coefficients for Estrone. Values greater than 0.995 are obtained. Figure 4 shows a typical calibration curve result for Estrone.
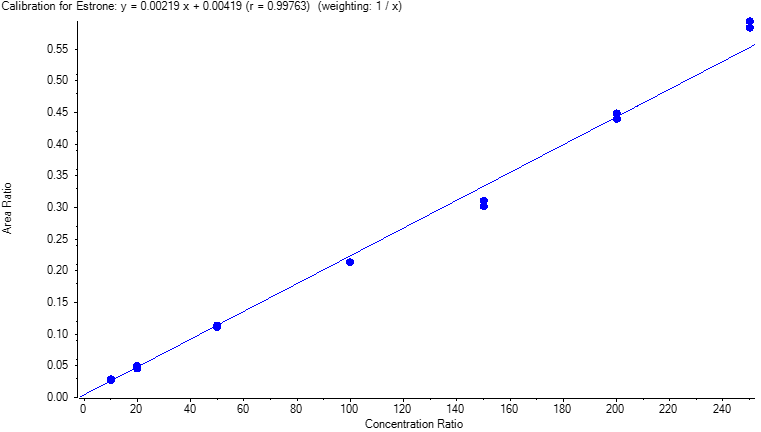
| R | |
|---|---|
| Curve 1 | 0.99763 |
| Curve 2 | 0.99848 |
| Curve 3 | 0.99748 |
| Curve 4 | 0.99811 |
Precision and Accuracy
For the accuracy and precision evaluation, the following acceptance criteria were used:
- Each concentration must not exceed 15% CV.
- Each concentration must be within 100 ± 15% of the nominal concentration.
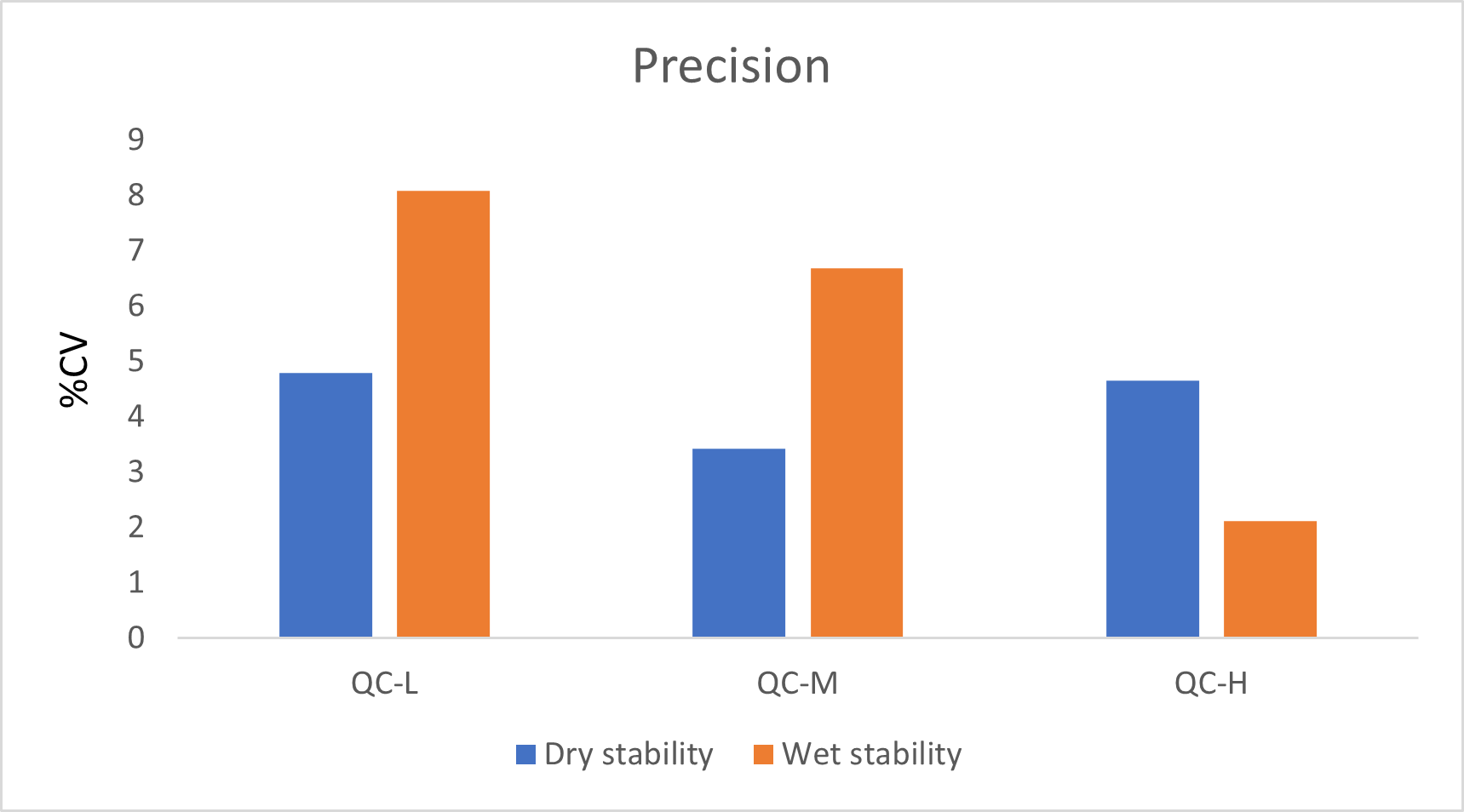
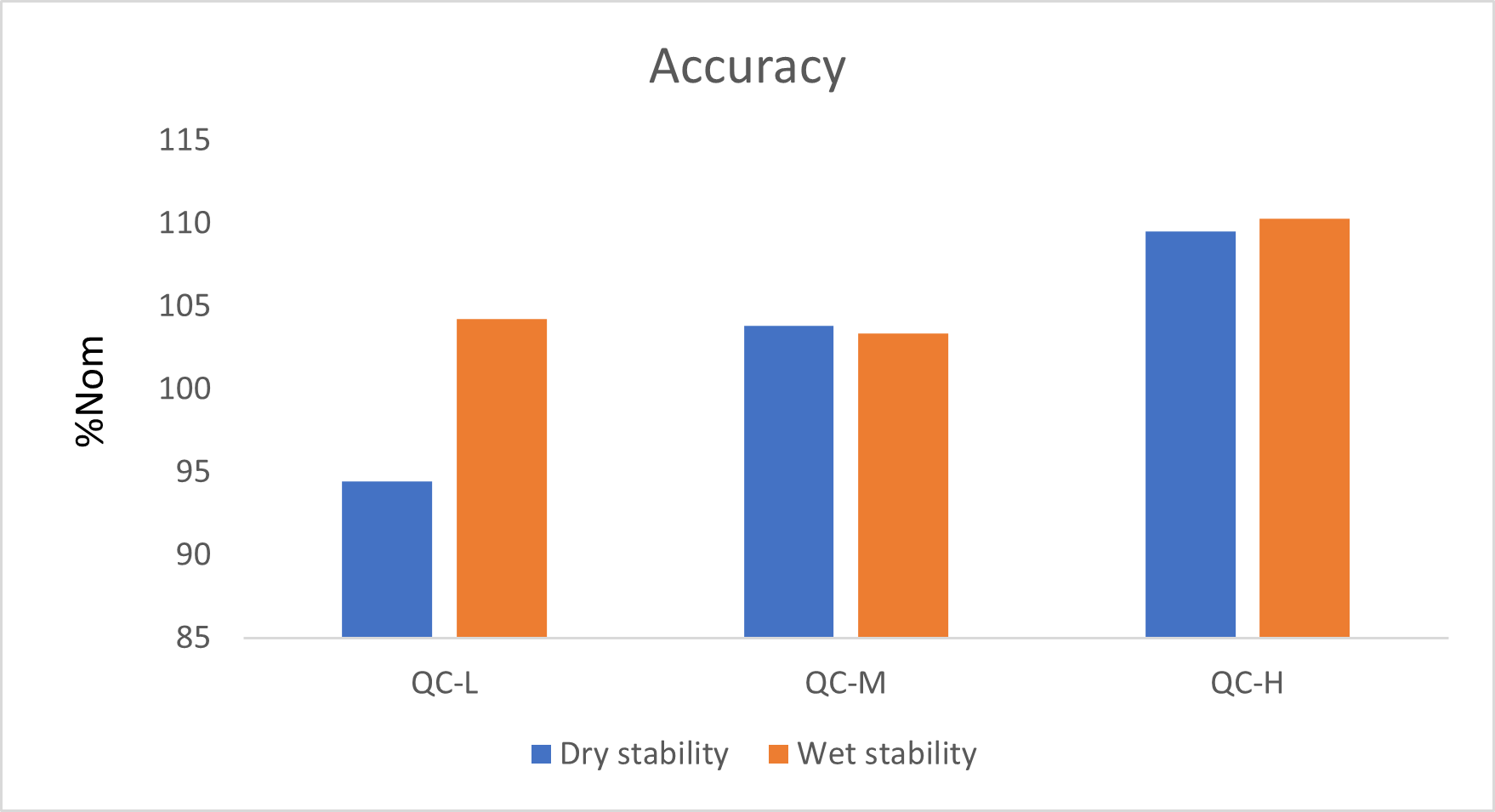
For the intra and inter-run precision and accuracy experiment, each replicate of QC was analyzed on four different runs. Table 3 and 4 show the intra and inter-run precision and accuracy results for Estrone. The obtained %CV was below 15% and the accuracy was within 15% of the nominal value.
| Estrone | LLOQ | QC-L | QC-M | QC-H | ULOQ |
|---|---|---|---|---|---|
| Conc (ng/mL) | 10 | 30 | 125 | 187.5 | 250 |
| N | 6 | 6 | 6 | 6 | 6 |
| Mean (ng/mL) | 10.6 | 31.0 | 115.3 | 196.2 | 261 |
| %CV | 12.9 | 5.5 | 3.7 | 3.1 | 4.1 |
| %Nom | 105.7 | 103.2 | 92.2 | 104.6 | 104.4 |
| Estrone | QC-L | QC-M | QC-H |
|---|---|---|---|
| Conc (ng/mL) | 30 | 125 | 187.5 |
| N | 15 | 15 | 15 |
| Mean (ng/mL) | 31.3 | 115.4 | 197.2 |
| %CV | 5.9 | 4.1 | 3.1 |
| %Nom | 104.2 | 92.3 | 105.2 |
Wet Stability of Sample Extracts
Following the extraction, sample extracts are kept at 4°C in closed containers. After 1 day, sample extracts are spotted on a LazWell™ plate, dried and analyzed. The precision and accuracy results of QCs are reported in Figure 5 and Figure 6. All the results are within the acceptable criteria range for 1 day at 4°C.
Dry Stability of Samples Spotted on LazWell™
Extracted samples are spotted onto a LazWell plate, dried and kept at room temperature for 2 hours before analysis. The precision and accuracy results of QCs are reported in Figure 5 and Figure 6. All the results are within the acceptable criteria range for 2 hours at room temperature.
Cross Validation Study
Serum samples from real patients were tested with this method to correlate with results obtained by traditional LC-MS/MS. The percentage difference between the values is evaluated. A difference of less than 20% is obtained. The results are reported in Table 5.
| LC
(pg/mL) |
Luxon
(pg/mL) |
%Diff | |
|---|---|---|---|
| M1 | 64.1 | 64.5 | 0.6 |
| M2 | 55.1 | 61.2 | 10.4 |
| M3 | 18.2 | 19.5 | 7.1 |
| M4 | 18.9 | 20.1 | 6.3 |
| M5 | 12.7 | 13.3 | 4.3 |
| M6 | 12.9 | 15.3 | 16.9 |
Conclusion
The Luxon Ion Source combined with the Sciex QTrap® 5500 mass spectrometer system enables the rapid quantification of free estrone in serum. This method of analysis can thus be applied at the clinical level in order to diagnose different estrone disorders.