Introduction
Toxicological labs need to screen different drugs classes like benzodiazepines, anxiolytics and anticonvulsants using a generic sample preparation and fast analytical technique. Benzodiazepines are a drug class that is prescribed as a tranquilizer and is often abused in conjunction with opioids and alcohol. Our goal for this application note is to optimize an automated sample preparation method for a benzodiazepine panel in urine using a single operation in LDTD®-MS/MS.
LDTD-MS/MS offers specificity combined with an ultra-fast analysis for an unrivaled screening method. To develop this application, we focused on performing a quick and simple sample preparation. Twenty-one drugs of the benzodiazepine class are analyzed simultaneously with quantitative screening results obtained in less than 8 seconds per sample. Each drug has been screened based on the industry cut-offs requirement.
Sample Preparation Method
Sample Collection
Urine samples were collected and transferred into barcoded tubes, readable by the Azeo Liquid Handler.

Sample Extraction (SALLE) : Panel 1
Each barcoded vial was scanned by the Azeo Liquid Handler and an automatic batch file was created. The Azeo extraction system (Figure 3) is used to extract the samples using the following conditions:
- 5 µL of Internal standard were added to each sample
- 75 µL of urine sample were transferred from the vials to a deep-well plate placed in the Lumo vortexer
- Mix
- 5 µL b-Glucuronidase-RT Enzyme/Hydrolysis buffer were added to each sample.
- Mix and incubate at room temperature for 15 minutes.
- 225 µL Extraction buffer and 300 µL Acetonitrile were added into the deep-well plate.
- Mix and centrifuge (5000 rpm) for 5 minutes for phase separation.
- Mix 6.5 µL of desorption buffer with 20 µL of upper layer phase.
- Spot 6 µL of the mixture onto a LazWell™96 plate
- Dry 5 minutes at 40°C
LDTD®-MS/MS Parameters
LDTD
Model: Luxon S-960, Phytronix
Carrier gas: 6.0 L/min (air)
Laser pattern:
- 6-second ramp to 55% power
- Hold 2 seconds at 55% power
MS/MS
MS model: QTrap® System 5500, Sciex
Scan Time: 5 msec
Ionization: APCI
Analysis Method: Positive MRM mode
| Drugs | Transition | CE |
|---|---|---|
| Meprobamate | 219.1 → 158.1 | 10 |
| Carisoprodol-d7 | 269.2 → 183.2 | 10 |
| Nordiazepam | 271.1 → 140.1 | 27 |
| 7-Aminoflunitrazepam | 284.0 → 226.0 | 50 |
| Diazepam | 285.1 → 154.1 | 32 |
| 7-AminoClonazepam | 286.1 → 222.2 | 30 |
| Oxazepam | 287.1 → 241.1 | 30 |
| 7-AminoClonazepam-d4 | 290.1 → 226.0 | 30 |
| 7-Aminoflunitrazepam-d7 | 291.1 → 230.0 | 50 |
| Oxazepam-d5 | 292.1 → 246.1 | 30 |
| Temazepam | 301.1 → 255.1 | 25 |
| Temazepam-d5 | 306.1 → 260.1 | 25 |
| Zaleplon | 306.1 → 265.2 | 20 |
| Zolpidem | 308.1 → 236.1 | 35 |
| Alprazolam | 309.1 → 274.1 | 35 |
| Alprazolam-d5 | 314.1 → 286.1 | 35 |
| Clonazepam | 316.0 → 214.0 | 50 |
| Lorazepam | 321.0 → 275.0 | 30 |
| (Alpha) Hydrozyalprazolam | 325.1 → 205.1 | 54 |
| Midazolam | 326.1 → 291.1 | 35 |
| Clozapine | 327.1 → 270.1 | 30 |
| 2-Hydroxyethylflurazepam | 333.1 → 194.1 | 30 |
| Alpha-OH-Midazolam | 342.1 → 203.1 | 35 |
| Etizolam | 343.1 → 314.0 | 25 |
| (Alpha) Hydroxytriazolam | 359.0 → 331.0 | 36 |
| Flurazepam | 388.1 → 315.0 | 27 |
Results and Discussion
Initial Cut-Off Test (ng/mL)
A drug list and screening cut-offs required by toxicological labs can be found in Table 2.
| Analyte | Cut-off (ng/mL) | Analyte | Cut-off (ng/mL) |
|---|---|---|---|
| (Alpha) Hydroxyalprazolam | 50 | Alprazolam | 50 |
| 7-Aminoclonazepam | 50 | Clozapine | 50 |
| Clonazepam | 50 | Etizolam | 50 |
| Diazepam | 50 | Flurazepam | 50 |
| Midazolam | 50 | Lorazepam | 50 |
| (Alpha) OH-Triazolam | 50 | Meprobamate | 100 |
| 2-Hydroxyethylflurazepam | 50 | Nordiazepam | 50 |
| Zaleplon | 50 | Oxazepam | 50 |
| 7-Aminoflunitrazepam | 50 | Temazepam | 50 |
| Alpha-OH-Midazolam | 50 | Zolpidem | 50 |
Precision / Accuracy
Spiked samples around the decision point (50% cut-off: QC-L, cut-off: CO and 200% cut-off: QC-H) and blank solutions are used to validate the precision of the method. The peak area against the internal standard (IS) ratio was used to normalize the signal. Replicate extractions are deposited onto a LazWell™ plate and dried before analysis.
The following acceptance criteria were used:
- Each concentration must not exceed 20% CV
- The mean concentration ± 2 times the standard deviation must not overlap with other concentrations at the cut-off.
For the inter-run precision experiment, each fortified sample set is analyzed in triplicate on five different days. Table 3 shows the inter-run precision results. No overlapping at the cut-off is observed for 2-Hydroxyethylflurazepam and the %CV was below 20%. Similar results are obtained for the other drugs in the panel.
| 2-Hydroxyethylflurazepam | QC-L | CO | QC-H |
|---|---|---|---|
| Conc (ng/mL) | 25 | 50 | 100 |
| N | 15 | 15 | 15 |
| Mean (ng/mL) | 22.9 | 51.2 | 103.9 |
| SD | 1.6 | 1.7 | 3.1 |
| %CV | 7.2 | 3.3 | 3.0 |
| Mean – 2SD (ng/mL) | 19.7 | 47.8 | 97.7 |
| Mean + 2SD (ng/mL) | 26.3 | 54.6 | 110.1 |
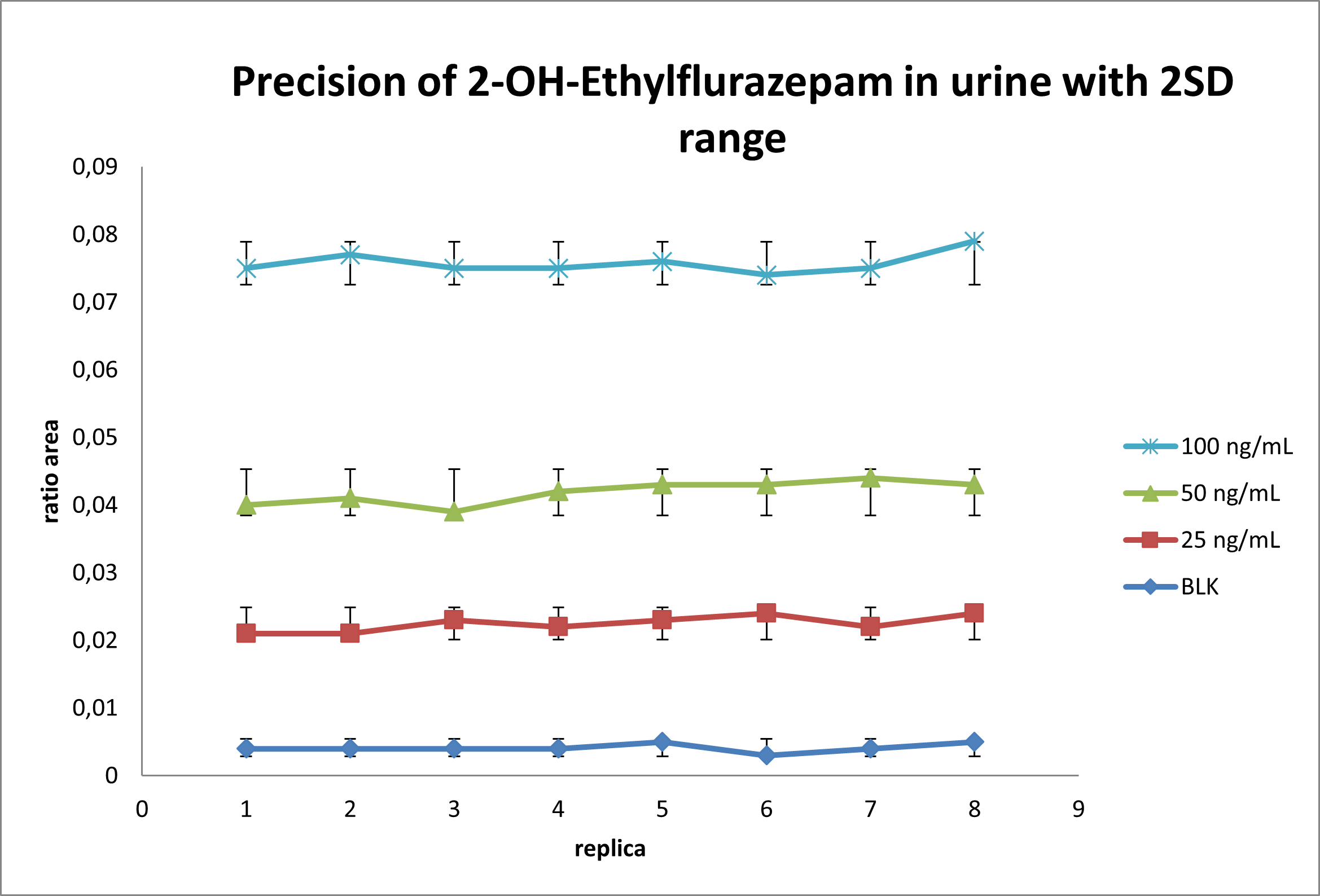
For the intra-run precision experiment, each fortified sample is extracted and analyzed in 8 replicates. Area ratio results are plotted using the ± 2 STD error bars. Figure 4 shows the intra-run results for 2-Hydroxyethylflurazepam. No overlapping is observed for each concentration and the %CV was below 20%. Similar results are obtained for the other drugs in the panel.
Multi-Matrix Validation
Urine samples were collected from 63 patients. Samples were analyzed using LC-MS/MS reference method and LDTD-MS/MS method. The method sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy are verified as followed:
|
LC-MS/MS |
|||
|---|---|---|---|
| Yes | No | ||
| LDTD-MS/MS | Yes | TP (True positive) | FP (False positive) |
| No | FN (False negative) | TN (True negative) | |
Where:
Sensitivity: (TP / (TP + FN))
Specificity: (TN / (TN + FP))
PPV: (TP / (TP + FP))
NPV: (TN / (TN + FN))
Accuracy: ((TP+TN) / (TP + FN+TN+FP))
Table 4 shows the analysis results of 63 real samples for 2-Hydroxyethylflurazepam.
| |
LC-MS/MS | ||
|---|---|---|---|
| Yes | No | ||
| LDTD-MS/MS | Yes | TP=8 | FP=0 |
| No | FN=0 | TN=55 | |
Validation results are reported in Table 5 for 2-Hydroxyethylflurazepam. Similar results are obtained for the other drugs.
| Parameters | 2-Hydroxyethylflurazepam |
|---|---|
| Sensitivity (%) | 100 |
| Specificity (%) | 100 |
| PPV (%) | 100 |
| NPV (%) | 100 |
| Accuracy (%) | 100 |
Conclusion
Luxon Ion Source® combined to a Sciex Q-Trap 5500 mass spectrometer system allows ultra-fast (8 seconds per sample) screening of a benzodiazepine panel in urine using a simple and automated sample preparation method.